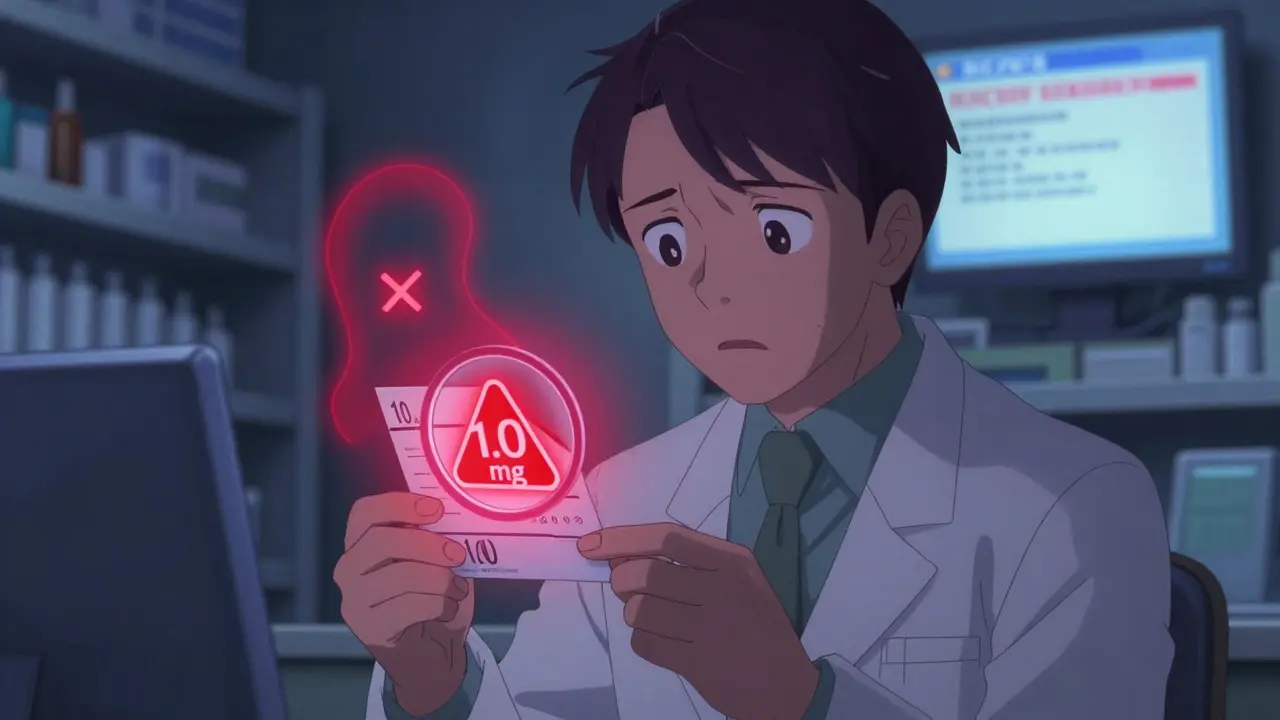
Transferring a prescription shouldn’t be a guessing game. Yet every day, patients get confused, pharmacists make mistakes, and dangerous errors slip through-like a patient receiving 10 times the intended dose because a label said ‘1.0 mg’ instead of ‘1 mg’. These aren’t hypothetical risks. They’re real, documented, and preventable. The good news? There’s a clear, regulated way to transfer prescriptions safely-and keep every label accurate from start to finish.
Why Prescription Transfers Go Wrong
Most errors happen because people assume transferring a prescription is like forwarding a text message. It’s not. A prescription isn’t just a note-it’s a legal document with strict rules. When a pharmacy receives a transfer request, they’re not just copying data. They’re verifying a chain of accountability that includes the prescriber, the original pharmacy, the patient, and the receiving pharmacy. One missing detail, one misread number, one outdated system, and you’re in danger territory.The biggest culprits? Trailing zeros. If a label says ‘5.0 mg’, that’s a red flag. The FDA and NCCMERP have tracked hundreds of cases where patients took 10 times the dose because someone misread ‘5.0’ as ‘50’. Leading zeros matter too. ‘.4 mg’ is a mistake. It must be ‘0.4 mg’. These aren’t suggestions-they’re federal requirements under 21 CFR § 201.100. And they’re not just about clarity. They’re about survival.
What the DEA Actually Allows in 2026
The rules changed in August 2023, and it was a big deal. Before then, Schedule II prescriptions-like oxycodone, fentanyl, or Adderall-could never be transferred between pharmacies. Now, they can. But only once. And only electronically.Here’s the breakdown:
- Controlled substances (Schedule II): One electronic transfer only. No refills can be transferred unless they were already authorized by the prescriber. After the transfer, the original prescription is void.
- Controlled substances (Schedule III-V): Can be transferred multiple times, up to the maximum refills allowed by the prescriber. Still must be electronic, unless under very limited exceptions.
- Non-controlled prescriptions: Most states allow multiple transfers, but electronic is still the safest and most accurate method.
And here’s the catch: You can’t fax, email, or call in a Schedule II transfer. The DEA requires full electronic transfer using the NCPDP SCRIPT 2017071 standard. Anything else is invalid. That means if your pharmacy still uses fax machines for controlled substances, they’re breaking federal law.
What Must Be on Every Prescription Label
Every label, whether printed or digital, must include these 10 elements-or it’s not legal:- Patient’s full name
- Drug name (generic or brand)
- Strength (in metric units only-no ‘grains’ or ‘minims’)
- Dosage form (tablet, capsule, liquid, etc.)
- Quantity dispensed
- Directions for use (e.g., ‘Take one tablet by mouth twice daily’)
- Prescriber’s name
- Prescription number
- Date issued
- Number of refills allowed
- Pharmacy name, address, and phone number
And here’s what’s not allowed:
- Abbreviations like ‘HCTZ’ for hydrochlorothiazide or ‘MOM’ for magnesium oxide
- Trailing zeros (‘1.0 mg’ → ‘1 mg’)
- Missing leading zeros (‘.4 mg’ → ‘0.4 mg’)
- Handwritten notes on labels unless from the prescriber
Why? Because in 2022, NCCMERP analyzed over 2,300 medication errors. Nearly 1 in 5 involved misread labels. The fix? Standardization. The FDA says standardized labeling could prevent 1.5 million adverse events a year.

How Transfers Actually Work (Step by Step)
It’s not magic. It’s a process. Here’s what happens when you request a transfer:- You call or visit the receiving pharmacy and ask to transfer your prescription. Never ask the old pharmacy to send it first-you might get stuck if they can’t.
- The receiving pharmacy checks if they have the medication in stock. If not, they won’t process the transfer. This step prevents delays.
- They send an electronic request using NCPDP SCRIPT standards. The original pharmacy receives it.
- The original pharmacy verifies the prescription is valid, checks remaining refills, and confirms the patient’s identity.
- They send back the full electronic record: drug, strength, refills, prescriber, DEA number, transfer date, and pharmacist ID.
- The receiving pharmacy prints or displays the label with all required info. They mark the record as ‘TRANSFERRED’ and note the original pharmacy’s details.
- The original pharmacy voids the prescription. No refills can be filled there anymore.
For Schedule II drugs, the receiving pharmacy gets only one shot. If they mess up the label or miss a refill count, the patient can’t go back to the original pharmacy. No second chances.
What Patients Must Do to Stay Safe
You’re not just a passive recipient-you’re part of the safety chain.- Always confirm the receiving pharmacy can fill the prescription before you request the transfer. A 2022 California Board of Pharmacy study found 23% of transfer attempts failed because patients didn’t check inventory first.
- Don’t assume your old pharmacy will notify you. They won’t. You have to initiate the transfer.
- Check the label when you pick it up. Compare the drug name, strength, and directions to what your doctor told you. If something looks off, ask.
- For controlled substances, plan ahead. Schedule II prescriptions can only be filled once after transfer. If you’re traveling or switching pharmacies, make sure you have enough supply to cover the gap.
Patients on Reddit’s r/pharmacy shared horror stories: one person transferred their oxycodone prescription, only to find the new pharmacy didn’t have it in stock. They went five days without pain relief. Another got the wrong strength because the label was truncated during transfer. Both could’ve been avoided with a simple phone call.

Technology and the Future of Label Accuracy
The best tool for preventing errors isn’t a person-it’s software. Pharmacies using NCPDP SCRIPT 2017071 have a 98.7% data accuracy rate, according to a 2022 University of Florida study. Fax? Only 82.3%. Phone? Just 76.1%.But not all systems are equal. Independent pharmacies often use outdated software. A 2022 National Community Pharmacists Association survey found 18% of pharmacies reported data truncation during transfers-meaning part of the label gets cut off. That’s how you end up with ‘1.0 mg’ instead of ‘1 mg’.
The next big shift? The FDA’s Patient Medication Information (PMI) rule, coming in 2025. It will require:
- Standardized label layouts
- Barcode scanning before dispensing
- Automated checks for trailing zeros, missing leading zeros, and incorrect units
- Electronic labels available on request, but paper as the default
Early adopters say it’s expensive-$12,500 to $18,750 per pharmacy to upgrade-but the payoff is clear. ASHP forecasts a 75% drop in transfer errors once pharmacies integrate with EHR systems like Epic and Cerner.
What Happens If You Get It Wrong?
The DEA issued 142 warning letters to pharmacies in 2022 for improper transfers-up 28% from 2021. Most were for Schedule II violations: transferring more than once, using fax, or altering prescription data.Pharmacists can lose their licenses. Pharmacies can lose their DEA registration. And patients? They could end up in the ER.
There’s no gray area. If a label says ‘1.0 mg’ and a patient takes it as ‘10 mg’, that’s not a mistake. It’s negligence. And it’s preventable.
Bottom Line: Accuracy Isn’t Optional
Prescription transfers are complex, but they don’t have to be dangerous. The rules are clear. The technology exists. The data is there.Whether you’re a patient, a pharmacist, or a caregiver, your job is simple: verify. Double-check the label. Confirm the pharmacy can fill it. Use electronic transfers. Avoid abbreviations. Never ignore a trailing zero.
One wrong digit can change a life. But one careful step can save it.

Graham Holborn
Hi, I'm Caspian Osterholm, a pharmaceutical expert with a passion for writing about medication and diseases. Through years of experience in the industry, I've developed a comprehensive understanding of various medications and their impact on health. I enjoy researching and sharing my knowledge with others, aiming to inform and educate people on the importance of pharmaceuticals in managing and treating different health conditions. My ultimate goal is to help people make informed decisions about their health and well-being.