Have you ever picked up a prescription and noticed the pill looks exactly like your brand-name drug-but the box says something totally different? Maybe it’s labeled "colchicine" instead of "Colcrys," or "methylphenidate ER" instead of "Concerta." You might wonder: Is this a fake? A cheaper knockoff? Or is it actually the same thing? The answer is simpler than you think: you’re holding an authorized generic.
What Exactly Is an Authorized Generic?
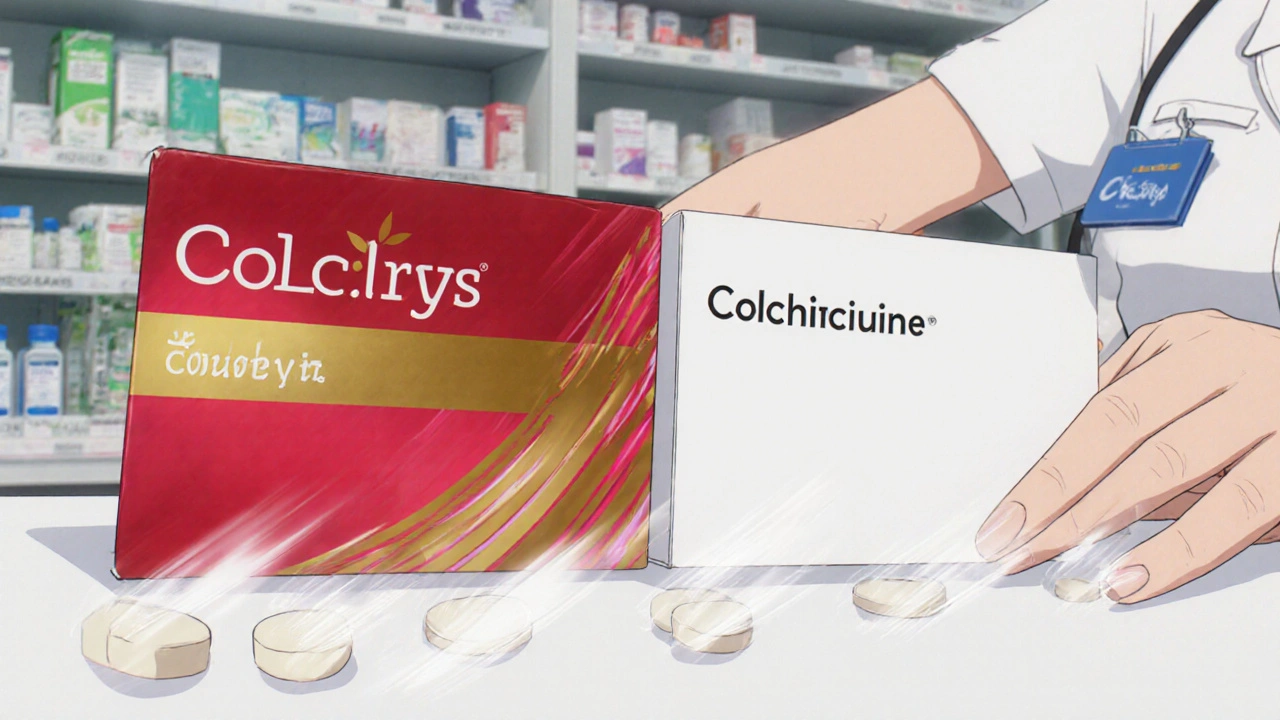
An authorized generic is the exact same medication as the brand-name drug you’re used to-same active ingredient, same inactive ingredients, same strength, same shape, same manufacturer. The only difference? It doesn’t have the brand name on the label. The U.S. Food and Drug Administration (FDA) defines it clearly: an authorized generic is an approved brand-name drug sold without the brand name on the packaging. That’s it. No changes to the formula. No shortcuts in production. It’s not a copy. It’s the real thing, just repackaged. These drugs first showed up in the U.S. market in the 1990s. By the 2010s, they became a major tool for pharmaceutical companies. Between 2010 and 2019, over 850 authorized generics hit the market. That’s not random. It’s strategy.How Are Authorized Generics Different From Regular Generics?
This is where things get confusing. Most people think all generics are the same. They’re not. Regular generics go through a process called the Abbreviated New Drug Application (ANDA). To get approved, the maker must prove their version works the same way as the brand-name drug. That means matching the active ingredient and showing it’s absorbed the same way in the body. But they’re allowed to use different fillers, dyes, or coatings. That’s why your generic version of a pill might be a different color or shape than the brand. Authorized generics skip all that. They’re made under the original brand’s New Drug Application (NDA). That means they don’t need to prove bioequivalence-they’re already proven. They’re made in the same factory, with the same equipment, using the exact same recipe. Even the inactive ingredients match. Here’s the kicker: authorized generics don’t show up in the FDA’s Orange Book, where traditional generics are listed. So if you’re looking for them in a pharmacy database, you won’t find them there. You’ll only see them when the pharmacist pulls them off the shelf.Who Makes Authorized Generics?
There are two ways this works. First, the brand-name company makes it themselves. They produce the exact same drug, then put it in plain packaging and sell it under a different name. For example, Pfizer made an authorized generic of Lipitor (atorvastatin) under the name "atorvastatin calcium." Same pill. Different box. Second, the brand-name company licenses the formula to another manufacturer. That manufacturer then produces the drug under contract. This is common with companies like Prasco Laboratories or Greenstone Pharmaceuticals. They don’t create the drug-they just make it exactly as the brand does. Either way, the drug inside is identical. No compromises.Why Do Companies Even Make Authorized Generics?
It sounds weird. Why would a company that spent millions developing a drug turn around and sell a cheaper version of it? The answer is competition. When a brand-name drug’s patent expires, other companies can make generics. That usually causes the price to drop-sometimes by 80% or more. The brand-name company loses money fast. So they launch an authorized generic. It’s still their drug. Same factory. Same quality. But now they’re selling it for less. And because it’s identical, pharmacies and insurers often prefer it over other generics. Studies show that in 75% of cases, authorized generics launch after a traditional generic has already entered the market. That’s not accidental. It’s a defensive move. They’re not trying to help you save money-they’re trying to keep you from switching to a competitor’s generic. In markets where a single generic company gets 180 days of exclusivity, about 70% of authorized generics launch before or during that window. That’s a tactic to steal market share before others can build momentum.
Are Authorized Generics Cheaper?
Yes-but not always as cheap as you’d hope. Typically, authorized generics cost 15% to 25% less than the brand-name version. That’s a solid saving. But they’re often more expensive than traditional generics that come later. Why? Because there’s less competition. If you’re the only generic option on the shelf, you can charge more. Once five other companies start making the same drug, prices crash. Authorized generics don’t always drop that far. For patients on high-cost medications like Concerta, Celebrex, or Unithroid, an authorized generic can mean saving $50 to $100 a month. That’s life-changing for people on fixed incomes. But if you’re shopping around and see a $10 generic version of your drug at Walmart, that’s probably a traditional generic. The authorized version might be $25. Still cheaper than the brand, but not the cheapest option.What Does This Mean for Patients?
From a medical standpoint, authorized generics are the safest generic option you can get. No changes in ingredients. No risk of different absorption rates. If you’ve had bad reactions to other generics because of fillers or coatings, an authorized generic might be the only version that works for you. But here’s the problem: you might not even know you’re getting one. Pharmacists often substitute generics automatically. If your insurance prefers the authorized version, they’ll give you that without asking. You might open your bottle and think, "This looks just like my old prescription," and not realize it’s a different label. Some patients get confused-or even suspicious. "Why is my doctor prescribing a generic version of my brand? Is this lower quality?" The answer is no. But you need to ask. If you’re on a medication that’s available as an authorized generic, ask your pharmacist: "Is this the same as the brand?" They’ll tell you. If you’re concerned about cost, ask: "Is there a cheaper generic available?"Why Aren’t Authorized Generics Listed in the Orange Book?
The FDA’s Orange Book is the official list of all approved generic drugs and their therapeutic equivalence ratings. It’s used by pharmacists, doctors, and insurers to decide what can be substituted. Authorized generics aren’t listed there because they’re not technically "generics" under FDA rules. They’re brand-name drugs sold under a different label. Since they don’t go through the ANDA process, they don’t qualify for inclusion. That makes tracking them harder. If you’re researching your drug, you won’t find it in the Orange Book. You have to check the FDA’s separate "List of Authorized Generic Drugs," which is updated periodically and not always easy to find.
Real Examples You Might Recognize
Here are a few common medications that have authorized generic versions:- Colcrys → Colchicine (made by Prasco)
- Concerta → Methylphenidate ER (made by Watson/Actavis)
- Celebrex → Celecoxib (made by Greenstone)
- Unithroid → Levothyroxine (made by Jerome Stevens)
- Lipitor → Atorvastatin Calcium (made by Pfizer)
What’s the Future of Authorized Generics?
They’re not going away. In fact, they’re growing. As more brand-name drugs lose patents, manufacturers will keep using authorized generics as a way to protect profits. They’re legal. They’re effective. And they give companies control over the transition from brand to generic. Some critics argue this slows down true competition. If the brand company controls the authorized version, they can delay the entry of cheaper generics by pricing the authorized version just low enough to keep insurers happy-but not low enough to let others compete. Regulators are watching. The FDA has no plans to change the rules, but lawmakers have raised questions about whether authorized generics undermine the spirit of the Hatch-Waxman Act, which was designed to increase generic competition. For now, they’re here to stay. And for patients who need stability, consistency, and reliability in their meds, they’re a quiet win.What Should You Do?
If you’re on a brand-name drug that’s been on the market for a few years:- Ask your pharmacist: "Is there an authorized generic for this?"
- Ask your doctor: "Would an authorized generic work for me?"
- Compare prices: Check your insurance copay for the brand, the authorized generic, and any other generics.
- If cost is a barrier, the authorized generic is often the best middle ground-better than the brand, more reliable than other generics.
Are authorized generics the same as brand-name drugs?
Yes. Authorized generics are identical to their brand-name counterparts in every way: same active ingredient, same inactive ingredients, same strength, same dosage form, same manufacturer, and same quality standards. The only difference is the label-no brand name appears on the packaging.
How are authorized generics different from regular generics?
Regular generics must prove they’re bioequivalent to the brand-name drug through the FDA’s ANDA process, and they can use different inactive ingredients. Authorized generics skip this process entirely-they’re made under the original brand’s approval (NDA) and use the exact same formula, down to the fillers and coatings.
Why aren’t authorized generics listed in the FDA’s Orange Book?
The Orange Book only includes drugs approved through the Abbreviated New Drug Application (ANDA) process. Authorized generics are marketed under the original brand’s New Drug Application (NDA), so they don’t qualify for inclusion. Instead, the FDA maintains a separate, less-publicized list of authorized generics.
Are authorized generics cheaper than brand-name drugs?
Yes, usually by 15% to 25%. But they’re often more expensive than traditional generics that enter the market later. That’s because authorized generics are sold by the original brand manufacturer or its partner, and there’s less competition. Once multiple generic makers enter, prices drop further.
Can I trust authorized generics if I’ve had bad reactions to other generics?
Yes. If you’ve had issues with other generics due to different inactive ingredients (like dyes, fillers, or coatings), an authorized generic is often the safest option. Since it uses the exact same formulation as the brand-name drug, it’s far less likely to cause unexpected reactions.
Why would a drug company make its own generic?
It’s a business strategy. When a patent expires, other companies can make cheaper generics. By launching their own authorized generic, the brand company keeps market share, keeps relationships with pharmacies and insurers, and avoids losing all revenue. It’s not about helping patients-it’s about controlling the transition.
How do I know if I’m getting an authorized generic?
Check the drug name on your prescription label. If it’s the generic version of a brand-name drug but the manufacturer is the same as the brand (e.g., Pfizer making atorvastatin instead of Lipitor), you’re likely getting an authorized generic. Ask your pharmacist to confirm. They can tell you based on the label and manufacturer code.

Graham Holborn
Hi, I'm Caspian Osterholm, a pharmaceutical expert with a passion for writing about medication and diseases. Through years of experience in the industry, I've developed a comprehensive understanding of various medications and their impact on health. I enjoy researching and sharing my knowledge with others, aiming to inform and educate people on the importance of pharmaceuticals in managing and treating different health conditions. My ultimate goal is to help people make informed decisions about their health and well-being.